Quadrupole Ion Trap MS
Overview;
Ion Trapping;
Ion Excitation and Ejection;
Multiple Stages of Mass Spectrometry (MSn);
Resolution;
Limitations;
Recent Developments;
A quadrupole ion trap is a sensitive and versatile mass spectrometer, roughly
the size of a tennis ball
[Paul, 1990,
March, 1997,
Jonscher, 1997].
The mass range of commercial LC-traps is well matched
to the range of m/z values typically generated from the electrospray ionization
process and the unit resolution provided throughout the mass range affords charge
state identification of multiply-charged peptide ions. Quadrupole ion trap mass
spectrometers can analyze peptides from a tryptic digest present at the 20-100
fmol level. Perhaps the greatest strength of the ion trap technique lies in the
ability to perform multiple stages of mass spectrometry, unlike a triple quadrupole
instrument or a standard TOF-MS. Up to 12 stages of tandem mass spectrometry
(MS12) have been performed using an ion trap, greatly increasing the amount of
structural information obtainable for a given molecule. Three hyperbolic electrodes,
consisting of a ring and two endcaps, form the core of this instrument.
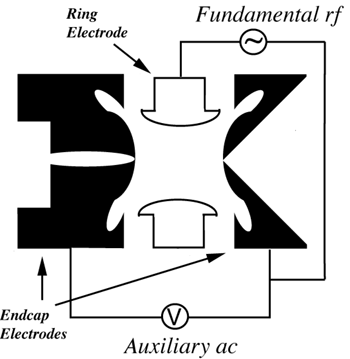
Transmission into the Trap:
Ions created by electrospray ionization are focused using an octupole transmission
system into the ion trap. Ions may be gated into the trap through the use of a pulsing
lens or through a combination of rf potentials applied to the ring electrode. The
pulsed transmission of ions into the trap differentiates ion traps from "beam"
instruments such as quadrupoles where ions continually enter the mass analyzer. The
time during which ions are allowed into the trap, termed the "ionization period"
, is set to maximize signal while minimizing
space-charge effects.
The ion trap is typically filled with helium to a pressure of about 1 mtorr.
Collisions with helium dampen the kinetic energy of the ions and serve to quickly
focus trajectories toward the center of the ion trap, enabling trapping of injected ions
[Louris, 1987,
Louris, 1989].
Stable Focusing of Ions in the Trap:
Trapped ions are further focused toward the center of the trap through the use of
an oscillating voltage, called the
fundamental rf,
applied to the ring electrode. An ion will be stably trapped depending upon
the values for the mass and charge of the ion, the radial size of the ion trap
(r), the oscillating frequency of the fundamental rf (w), and the amplitude of
the voltage on the ring electrode (V). The dependence of ion motion on these
parameters is described by the dimensionless parameter
qz:
qz = 4eV/mr2w2
An analogous parameter az describes the effect on the
motion of ions when a DC potential is applied to the ring electrode.
In order to create an ideal quadrupole field, r2=2z2
is required, where z is the axial distance from the center of the trap to the
endcap electrode. A number of groups over the past several years have discovered
that, by moving the electrodes farther apart or changing the asymptotic angle
between the electrodes, the higher multipole fields inside the trap may be accessed.
Use of these fields has led to increases in performance such as higher resolution
and more efficient ion excitation and ejection.
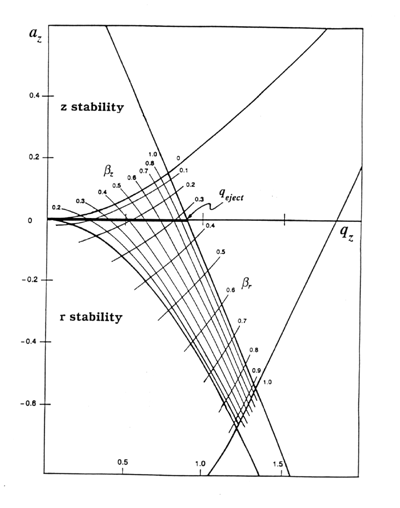
The "stability diagram" shows a theoretical region where radial and
axial stability overlap. Depending upon the amplitude of the voltage placed on
the ring electrode, an ion of a given m/z will have az,
qz values that will fall
within the boundaries of the stability diagram, and the ion will be trapped. If the az, qz values at that voltage falls outside
of the boundaries of the stability diagram, the ion will hit the electrodes and
be lost. Commercial ion traps work along the line az=0.
Mass Range for Trapping Ions:
Ions of different m/z values may have stable orbits at the same time.
Because ion trajectories become unstable at a particular value for
qeject, a well-defined low-mass cutoff is created for a given value
of the amplitude of the applied rf voltage, V. No ions below that mass will
be trapped, but ions above that mass will be trapped with trapping efficiency
decreasing for larger m/z values. The high mass cutoff for detection depends
in part on the amplitude and frequency of an
auxiliary potential
placed on the endcap electrodes. In this manner, the nominal mass range of the
instrument has been extended to m/z 4,000 - 6,000
[Kaiser, 1989].
This mass range is
considerably higher than that typically obtainable on a quadrupole instrument but
lower than that achievable on a time-of-flight mass spectrometer (TOF-MS).
Ejection and Detection:
Ions oscillate in the trap with a frequency known as the
secular frequency
that is determined by the values for az and qz
and by the frequency of the fundamental rf. A mass spectrum is generated by sequentially
ejecting fragment ions from low m/z to high m/z. This is done by
scanning the amplitude of the fundamental rf voltage to make ion trajectories
sequentially become unstable. Ions are ejected through holes in the endcap electrode
and detected using a collision dynode and electron multiplier system
[Stafford, 1984].
Resonance Ejection:
Resonance
conditions are induced by matching the frequency of a
supplementary potential
applied on the endcap electrodes to the secular frequency (as determined by
qz) of the ion.
If the amplitude of the resonance signal is large enough, ions will be ejected from the trap.
Resonance ejection
occurs at qz values lower than those typically required for ejection at
qeject. Since qz is inversely proportional to
m, ions of larger molecular weight can be ejected under resonance conditions.
Resonance Excitation:
Individual ions are isolated by the application of a waveform signal across the endcap
electrodes. Structural information is obtained by the application of a low
amplitude resonance signal termed the
tickle voltage
across the endcap electrodes. The ion motion between the endcaps increases,
leading to ion dissociation due to thousands of collisions with the helium
damping gas.
This process causes random fragmentation along the peptide backbone in a manner
analogous to that obtained using a triple quadrupole mass spectrometer.
FTICR and quadrupole ion traps are unique in their ability to perform multiple
stages of mass spectrometry, enormously increasing the amount of information
obtainable from a molecule. For both types of instruments, waveforms are
constructed to isolate an ion, induce its fragmentation, then isolate one of
the products, induce its fragmentation, etc. Finally, the resultant ions
from all of the manipulations are ejected from the trap and detected
[Soni, 1994]. Typically
a maximum of 3 stages of mass spectrometry are performed for peptide analysis
[Louris, 1990].
The mass resolution
of the ion trap is a function of the number of rf cycles that the ion spends
interacting with the trapping field. Resolution is increased to provide
charge state determination for multiply-charged peptide ions by decreasing
the scan speed of the ejection voltage. Typical mass resolutions can be
unit or better depending on the scan speed of the instrument and the width
of the mass window investigated. Mass resolutions as high as 30,000 have
been reported but are not typical
[Cooks, 1992].
Charge-state determination is not
possible using a triple quadrupole mass spectrometer under the conditions
normally used for high sensitivity peptide analysis.
Click here
for more information about resolution.
- A limitation of the ion trap is that the alternate scan modes of triple
quadrupole mass spectrometers, such as precursor ion and neutral loss scans,
are currently not possible. These scan modes are particularly useful for identifying
the presence of trace components in complex mixtures.
- The upper limit on the ratio between the precursor mass and the lowest trapped
fragment ion mass is approximately 0.3 (dependent on the qz value). The fragment
ions with masses in the lower third of the mass range will not be detected,
therefore the first several b- and y-type
fragment ions
may not be observed for a given peptide. The newly-released tandem Q-TOFs have
improved capability for performing de novo sequencing since all fragment ions
may be detected at high resolution.
- The dynamic range of ion traps is limited because, when there are too many
ions in the trap, space charge effects lead to diminished performance. Automated
scans are used to rapidly count the ions before they go into the trap so that the
time ions are allowed to enter the trap is dependent on the ion flux. This
ensures only a certain number of ions get in. This can be a problem when
trace elements in particularly dirty matrices are analyzed because the trap
fills with both matrix ions (large number) and trace sample ions (very small
number).
- ESI sources generate multiply-charged ions, making molecular weight
assignment difficult when peptide mixtures are analyzed off-line. Several
groups constructed MALDI-ion traps in an effort to simplify off-line
mixture analysis by generating singly-charged molecules. These traps
have been found to be particularly useful in phosphopeptide analysis.
For example, pSer and pThr were observed to routinely lose 98 amu from
the molecular ion, while pTyr lost 80 amu. The difference between the
mass of the phosphorylated molecular ion and its unphosphorylated analog
provided a fingerprint to help confirm the presence of a phosphopeptide
in the mixture.
- An interesting configuration utilizes the MSn capabilities
of ion traps and the rapid scanning of TOFs to create very powerful hybrid
mass spectrometers
[Chen, 1999,
Huang, 2000].
These IT-reTOFs, coupled with capillary LC ion sources,
provide an alternative method to identify proteins from complex mixtures.
Since gel electrophoresis is not required, sample losses due to gel-related
restrictions and peptide extraction post-digestion are minimized. Typically,
more protein coverage is obtained by this technique than is observed when
conventional MALDI-TOF is used
[He, 1997].
- The role of quadrupole ion traps is expanding from traditional pharmaceutical
applications into the revolutionary field of proteomics, where identification of
native and post-translationally modified proteins provides insight into complex
biological systems
[Haynes, 2000].
Although most proteomics experiments involve the use of
MALDI-TOF to screen tryptic digests of in gel digested proteins, the ion trap
has proven itself in its ability to generate high sensitivity fragmentation
spectra when the MALDI method gives inconclusive identification
[Qin, 1997 #356].
|